An easy and cheap procedure to immobilize TiO2 on glass surfaces using TiO2/SiO2 nanocomposite: Characterization and performance for the degradation of micropollutants of emerging concern in aqueous solutions
DOI:
https://doi.org/10.52493/j.cote.2021.1.10Keywords:
Titanium dioxide, silicon dioxide, heterogeneous photocatalysis, compound parabolic concentrator, emerging contaminantsAbstract
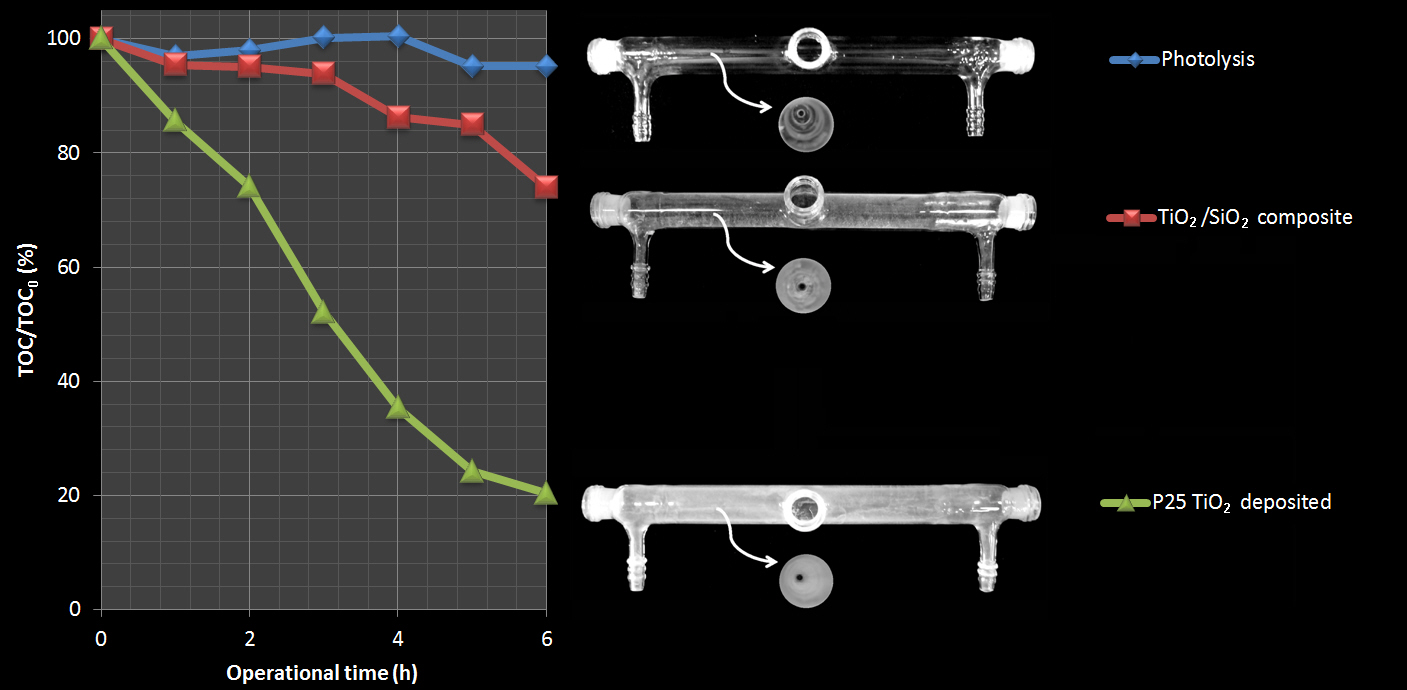
A simple high area TiO2/SiO2 nanocomposite was synthesized, characterized, and used to support P25 TiO2 on glass surfaces leading to an ease and cheap way to promote adhesion without refined pre-treatment steps. Photocatalytic performance and stability of the immobilized TiO2 was evaluated in laboratorial and pilot scales, under artificial and solar lights. Degradation rates of 80% and 45% were obtained for salicylic acid (16 mg L-1) and 17β-estradiol (E2, 1.0 mg L-1), respectively, after 4 h using a lab-made annular reactor. Solar batch experiments show a degradation rate of 85% for E2 (10 µg L-1) after 90 min. Photodegradation of trimethoprim (TMP, 500 ng L-1) and levofloxacin (LEVO, 1.0 mg L-1) using a compound parabolic concentrator (CPC) solar reactor revealed removal rates of 50% (once-through experiment) and 95% (batch experiment), respectively. CPC experiments show that the coated composite presents high physical stability after innumerous reuse cycles (more than 2000) under vigorous flow in continuous and batch operation. Overall, results evidenced the efficacy of the TiO2/SiO2 composite coated with P25 TiO2 on the degradation of micropollutants of emerging concern with low energetic costs.
References
Aguado, J., van Grieken, R., López-Muñoz, M.-J., & Marugán, J. (2006). A comprehensive study of the synthesis, characterization and activity of TiO2 and mixed TiO2/SiO2 photocatalysts. Applied Catalysis A: General, 312, 202–212. https://doi.org/10.1016/j.apcata.2006.07.003
Bideau, M., Claudel, B., Dubien, C., Faure, L., & Kazouan, H. (1995). On the “immobilization” of titanium dioxide in the photocatalytic oxidation of spent waters. Journal of Photochemistry and Photobiology A: Chemistry, 91(2), 137–144. https://doi.org/10.1016/1010-6030(95)04098-Z
Chang, H.-S., Choo, K.-H., Lee, B., & Choi, S.-J. (2009). The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. Journal of Hazardous Materials, 172(1), 1–12. https://doi.org/10.1016/j.jhazmat.2009.06.135
Chen, W. R., Sharpless, C. M., Linden, K. G., & Suffet, I. H. (2006). Treatment of volatile organic chemicals on the EPA Contaminant Candidate List using ozonation and the O3/H2O2 advanced oxidation process. Environmental Science & Technology, 40(8), 2734–2739. https://doi.org/10.1021/es051961m
Cho, M., Chung, H., Choi, W., & Yoon, J. (2004). Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Research, 38(4), 1069–1077. https://doi.org/10.1016/j.watres.2003.10.029
Coleman, H. M., Eggins, B. R., Byrne, J. A., Palmer, F. L., & King, E. (2000). Photocatalytic degradation of 17-β-oestradiol on immobilised TiO2. Applied Catalysis B: Environmental, 24(1), L1–L5. https://doi.org/10.1016/S0926-3373(99)00091-0
Dijkstra, M. F. J., Michorius, A., Buwalda, H., Panneman, H. J., Winkelman, J. G. M., & Beenackers, A. A. C. . (2001). Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catalysis Today, 66(2), 487–494. https://doi.org/10.1016/S0920-5861(01)00257-7
Esplugas, S., Giménez, J., Contreras, S., Pascual, E., & Rodrı́guez, M. (2002). Comparison of different advanced oxidation processes for phenol degradation. Water Research, 36(4), 1034–1042. https://doi.org/10.1016/S0043-1354(01)00301-3
Everett, D. H. (1972). Manual of symbols and terminology for physicochemical quantities and units, Appendix II: definitions, terminology and symbols in colloid and surface chemistry. Pure and Applied Chemistry, 31(4), 577–638. https://doi.org/10.1351/pac197231040577
Faisal, M., Abu Tariq, M., & Muneer, M. (2007). Photocatalysed degradation of two selected dyes in UV-irradiated aqueous suspensions of titania. Dyes and Pigments, 72(2), 233–239. https://doi.org/10.1016/j.dyepig.2005.08.020
Farreras, J. G., & Curcó, D. (2001). Modelos cinéticos y de radiación en sistemas fotocatalíticos. In M. A. Blesa (Ed.), Eliminación de contaminantes por fotocatálisis heterogênea (pp. 189–199). Red CYTED VIII-G.
Fox, M. A., & Dulay, M. T. (1993). Heterogeneous photocatalysis. Chemical Reviews, 93(1), 341–357. https://doi.org/10.1021/cr00017a016
Garcia-Rodríguez, A., Sagristà, E., Matamoros, V., Fontàs, C., Hidalgo, M., & Salvadó, V. (2014). Determination of pharmaceutical compounds in sewage sludge using a standard addition method approach. International Journal of Environmental Analytical Chemistry, 94(12), 1199–1209. https://doi.org/10.1080/03067319.2014.921292
Gaya, U. I., & Abdullah, A. H. (2008). Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 9(1), 1–12. https://doi.org/10.1016/j.jphotochemrev.2007.12.003
Gogate, P. R., & Pandit, A. B. (2004). A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Advances in Environmental Research, 8(3), 501–551. https://doi.org/10.1016/S1093-0191(03)00032-7
Guillard, C., Beaugiraud, B., Dutriez, C., Herrmann, J.-M., Jaffrezic, H., Jaffrezic-Renault, N., & Lacroix, M. (2002). Physicochemical properties and photocatalytic activities of TiO2-films prepared by sol–gel methods. Applied Catalysis B: Environmental, 39(4), 331–342. https://doi.org/10.1016/S0926-3373(02)00120-0
Guillard, C., Disdier, J., Herrmann, J.-M., Lehaut, C., Chopin, T., Malato, S., & Blanco, J. (1999). Comparison of various titania samples of industrial origin in the solar photocatalytic detoxification of water containing 4-chlorophenol. Catalysis Today, 54(2), 217–228. https://doi.org/10.1016/S0920-5861(99)00184-4
Hoffmann, M. R., Martin, S. T., Choi, W., & Bahnemann, D. W. (1995). Environmental applications of semiconductor photocatalysis. Chemical Reviews, 95(1), 69–96. https://doi.org/10.1021/cr00033a004
Howe, P. G., Benton, D. P., & Puddington, I. E. (1955). London-Van der Waals attractive forces between glass surfaces. Canadian Journal of Chemistry, 33(9), 1375–1383. https://doi.org/10.1139/v55-165
Keshmiri, M., Mohseni, M., & Troczynski, T. (2004). Development of novel TiO2 sol–gel-derived composite and its photocatalytic activities for trichloroethylene oxidation. Applied Catalysis B: Environmental, 53(4), 209–219. https://doi.org/10.1016/j.apcatb.2004.05.016
Li, S., Liu, J., & Feng, T. (2007). Low temperature coating of anatase thin films on silica glass fibers by liquid phase deposition. Journal of Wuhan University of Technology-Mater. Sci. Ed., 22(1), 136–139. https://doi.org/10.1007/s11595-005-1136-9
Liu, B., & Liu, X. (2004). Direct photolysis of estrogens in aqueous solutions. Science of The Total Environment, 320(2), 269–274. https://doi.org/10.1016/j.scitotenv.2003.08.005
Locatelli, M. A. F., Sodré, F. F., & Jardim, W. F. (2011). Determination of antibiotics in Brazilian surface waters using liquid chromatography-electrospray tandem mass spectrometry. Archives of Environmental Contamination and Toxicology, 60(3), 385–393. https://doi.org/10.1007/s00244-010-9550-1
Mercê, A. L. R., Lopes, P. P., Mangrich, A. S., & Levy, N. M. (2006). Molybdenum (VI) binded to humic and nitrohumic acid models in aqueous solutions. Salicylic, 3-nitrosalicylic, 5-nitrosalicylic and 3,5 dinitrosalicylic acids: part 2. Journal of the Brazilian Chemical Society, 17, 482–490. https://doi.org/10.1590/S0103-50532006000300008
Mikula, M., Brezová, V., Cěppan, M., Pach, L., & Karpinský, Ĺ. (1995). Comparison of photocatalytic activity of sol-gel TiO2 and P25 TiO2 particles supported on commercial fibreglass fabric. Journal of Materials Science Letters, 14(9), 615–616. https://doi.org/10.1007/BF00586156
Mitzi, D. B. (2004). Solution-processed inorganic semiconductors. Journal of Materials Chemistry, 14(15), 2355–2365. https://doi.org/10.1039/B403482A
Murashkevich, A. N., Lavitskaya, A. S., Barannikova, T. I., & Zharskii, I. M. (2008). Infrared absorption spectra and structure of TiO2-SiO2 composites. Journal of Applied Spectroscopy, 75(5), 730. https://doi.org/10.1007/s10812-008-9097-3
Ng, C. J. W., Gao, H., & Yang Tan, T. T. (2008). Atomic layer deposition of TiO2 nanostructures for self-cleaning applications. Nanotechnology, 19(44), 445604. https://doi.org/10.1088/0957-4484/19/44/445604
Nikolaou, A., Meric, S., & Fatta, D. (2007). Occurrence patterns of pharmaceuticals in water and wastewater environments. Analytical and Bioanalytical Chemistry, 387(4), 1225–1234. https://doi.org/10.1007/s00216-006-1035-8
Nishikawa, H., & Takahara, Y. (2001). Adsorption and photocatalytic decomposition of odor compounds containing sulfur using TiO2/SiO2 bead. Journal of Molecular Catalysis A: Chemical, 172(1), 247–251. https://doi.org/10.1016/S1381-1169(01)00124-8
Ohko, Y., Iuchi, K., Niwa, C., Tatsuma, T., Nakashima, T., Iguchi, T., Kubota, Y., & Fujishima, A. (2002). 17β-estradiol degradation by TiO2 photocatalysis as a means of reducing estrogenic activity. Environmental Science & Technology, 36(19), 4175–4181. https://doi.org/10.1021/es011500a
Paschoalino, M. P., Kiwi, J., & Jardim, W. F. (2006). Gas-phase photocatalytic decontamination using polymer supported TiO2. Applied Catalysis B: Environmental, 68(1), 68–73. https://doi.org/10.1016/j.apcatb.2006.08.001
Pereira, L. C., de Souza, A. O., Bernardes, M. F. F., Pazin, M., Tasso, M. J., Pereira, P. H., & Dorta, D. J. (2015). A perspective on the potential risks of emerging contaminants to human and environmental health. Environmental Science and Pollution Research, 22(18), 13800–13823. https://doi.org/10.1007/s11356-015-4896-6
Permpoon, S., Houmard, M., Riassetto, D., Rapenne, L., Berthomé, G., Baroux, B., Joud, J. C., & Langlet, M. (2008). Natural and persistent superhydrophilicity of SiO2/TiO2 and TiO2/SiO2 bi-layer films. Thin Solid Films, 516(6), 957–966. https://doi.org/10.1016/j.tsf.2007.06.005
Rosenfeldt, E. J., & Linden, K. G. (2004). Degradation of endocrine disrupting chemicals bisphenol a, ethinyl estradiol, and estradiol during UV photolysis and advanced oxidation processes. Environmental Science & Technology, 38(20), 5476–5483. https://doi.org/10.1021/es035413p
Rusu, C. N., & Yates, J. T. (2001). N2O adsorption and photochemistry on high area TiO2 powder. The Journal of Physical Chemistry B, 105(13), 2596–2603. https://doi.org/10.1021/jp0040345
Singh, H. K., Saquib, M., Haque, M. M., Muneer, M., & Bahnemann, D. W. (2007). Titanium dioxide mediated photocatalysed degradation of phenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid, in aqueous suspensions. Journal of Molecular Catalysis A: Chemical, 264(1), 66–72. https://doi.org/10.1016/j.molcata.2006.08.088
Sodré, F. F., Pescara, I. C., Montagner, C. C., & Jardim, W. F. (2010). Assessing selected estrogens and xenoestrogens in Brazilian surface waters by liquid chromatography-tandem mass spectrometry. Microchemical Journal, 96(1), 92–98. https://doi.org/10.1016/j.microc.2010.02.012
Sodré, F. F., & Sampaio, T. R. (2020). Development and application of a SPE-LC-QTOF method for the quantification of micropollutants of emerging concern in drinking waters from the Brazilian capital. Emerging Contaminants, 6, 72–81. https://doi.org/10.1016/j.emcon.2020.01.001
Takeda, S., Suzuki, S., Odaka, H., & Hosono, H. (2001). Photocatalytic TiO2 thin film deposited onto glass by DC magnetron sputtering. Thin Solid Films, 392(2), 338–344. https://doi.org/10.1016/S0040-6090(01)01054-9
Tolboom, S. N., Carrillo-Nieves, D., de Jesús Rostro-Alanis, M., de la Cruz Quiroz, R., Barceló, D., Iqbal, H. M. N., & Parra-Saldivar, R. (2019). Algal-based removal strategies for hazardous contaminants from the environment – A review. Science of The Total Environment, 665, 358–366. https://doi.org/10.1016/j.scitotenv.2019.02.129
Trung, T., & Ha, C.-S. (2004). One-component solution system to prepare nanometric anatase TiO2. Materials Science and Engineering: C, 24(1), 19–22. https://doi.org/10.1016/j.msec.2003.09.004
Xu, L. P., Zhao, Y. X., Wu, Z. G., & Liu, D. S. (2003). A new method for preparing Ti-Si mixed oxides. Chinese Chemical Letter, 14(11), 1159–1162. https://doi.org/http://www.qmtsg.com/qikan/726c45619f5c8c68ba3b61106b8ff674.html
Yu, H.-F., & Wang, S.-M. (2000). Effects of water content and pH on gel-derived TiO2–SiO2. Journal of Non-Crystalline Solids, 261(1), 260–267. https://doi.org/10.1016/S0022-3093(99)00658-4
Zhang, Y., Zhou, J. L., & Ning, B. (2007). Photodegradation of estrone and 17β-estradiol in water. Water Research, 41(1), 19–26. https://doi.org/10.1016/j.watres.2006.09.020
Downloads
Published
Issue
Section
License
Copyright (c) 2021 Matheus P. Paschoalino, Flávia C. S. Paschoalino, Wilson F. Jardim, Fernando Fabriz Sodré

This work is licensed under a Creative Commons Attribution 4.0 International License.


